Valentine’s Day Reading

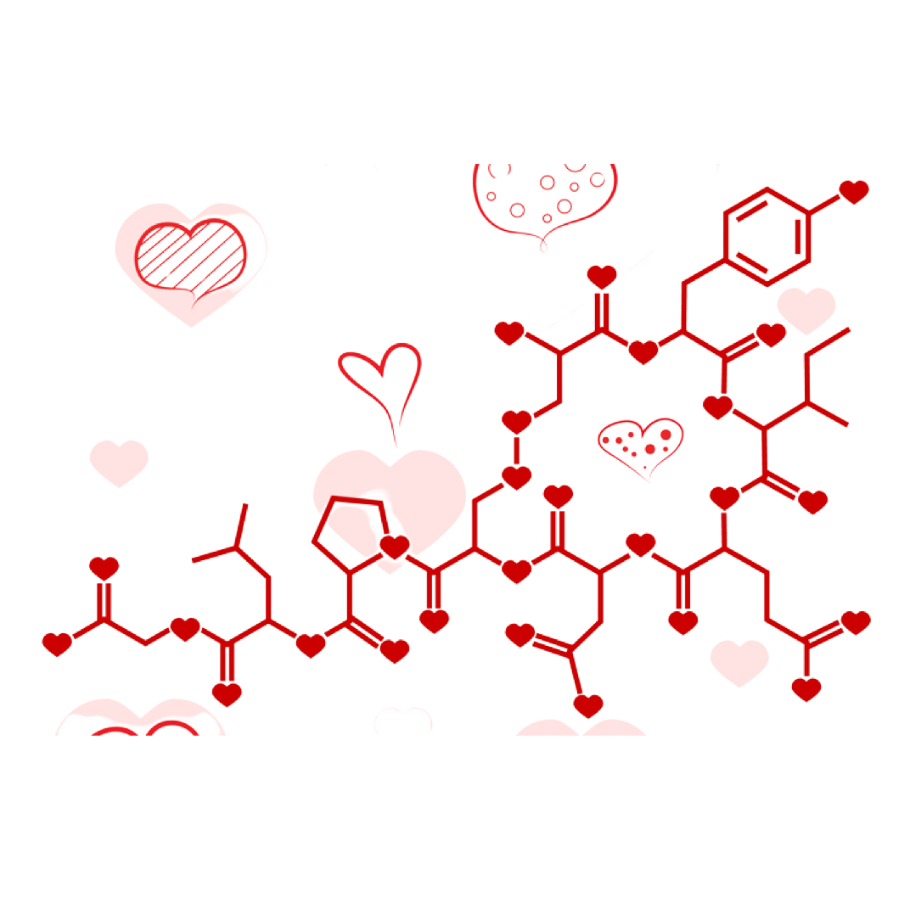
C8H11NO2 + C10H12N2O + C43H66N12O12S2 or in other words Happy Valentine’s Day!
Fetal whole heart blood flow imaging using 4D cine MRI
Prenatal detection of congenital heart disease facilitates the opportunity for potentially lifesaving care immediately after the baby is born. Echocardiography is routinely used for screening of morphological malformations, but functional measurements of blood flow are scarcely used in fetal echocardiography due to technical assumptions and issues of reliability. Magnetic resonance imaging (MRI) is readily used for quantification of abnormal blood flow in adult hearts, however, existing in utero approaches are compromised by spontaneous fetal motion. Here, we present and validate a novel method of MRI velocity-encoding combined with a motion-robust reconstruction framework for four-dimensional visualization and quantification of blood flow in the human fetal heart and major vessels. We demonstrate simultaneous 4D visualization of the anatomy and circulation, which we use to quantify flow rates through various major vessels. The framework introduced here could enable new clinical opportunities for assessment of the fetal cardiovascular system in both health and disease.
Fabrication and In Vitro Characterization of a Tissue Engineered PCL-PLLA Heart Valve
Heart valve diseases are among the leading causes of cardiac failure around the globe. Nearly 90,000 heart valve replacements occur in the USA annually. Currently, available options for heart valve replacement include bioprosthetic and mechanical valves, both of which have severe limitations. Bioprosthetic valves can last for only 10–20 years while patients with mechanical valves always require blood-thinning medications throughout the remainder of the patient’s life. Tissue engineering has emerged as a promising solution for the development of a viable, biocompatible and durable heart valve; however, a human implantable tissue engineered heart valve is yet to be achieved. In this study, a tri-leaflet heart valve structure is developed using electrospun polycaprolactone (PCL) and poly L-lactic acid (PLLA) scaffolds, and a set of in vitro testing protocol has been developed for routine manufacturing of tissue engineered heart valves. Stress-strain curves were obtained for mechanical characterization of different valves. The performances of the developed valves were hemodynamically tested using a pulse duplicator, and an echocardiography machine. Results confirmed the superiority of the PCL-PLLA heart valve compared to pure PCL or pure PLLA. The developed in vitro test protocol involving pulse duplicator and echocardiography tests have enormous potential for routine application in tissue engineering of heart valves.
Regulation of cardiomyocyte fate plasticity: a key strategy for cardiac regeneration
With the high morbidity and mortality rates, cardiovascular diseases have become one of the most concerning diseases worldwide. The heart of adult mammals can hardly regenerate naturally after injury because adult cardiomyocytes have already exited the cell cycle, which subseqently triggers cardiac remodeling and heart failure. Although a series of pharmacological treatments and surgical methods have been utilized to improve heart functions, they cannot replenish the massive loss of beating cardiomyocytes after injury. Here, we summarize the latest research progress in cardiac regeneration and heart repair through altering cardiomyocyte fate plasticity, which is emerging as an effective strategy to compensate for the loss of functional cardiomyocytes and improve the impaired heart functions. First, residual cardiomyocytes in damaged hearts re-enter the cell cycle to acquire the proliferative capacity by the modifications of cell cycle-related genes or regulation of growth-related signals. Additionally, non-cardiomyocytes such as cardiac fibroblasts, were shown to be reprogrammed into cardiomyocytes and thus favor the repair of damaged hearts. Moreover, pluripotent stem cells have been shown to transform into cardiomyocytes to promote heart healing after myocardial infarction (MI). Furthermore, in vitro and in vivo studies demonstrated that environmental oxygen, energy metabolism, extracellular factors, nerves, non-coding RNAs, etc. play the key regulatory functions in cardiac regeneration. These findings provide the theoretical basis of targeting cellular fate plasticity to induce cardiomyocyte proliferation or formation, and also provide the clues for stimulating heart repair after injury.
microRNA protects the heart
When the supply of oxygen to the heart is insufficient to meet metabolic demands, myocardial cells undergo apoptosis, which can cause myocardial infarction. Mitochondrial fission is known to play a part in this process, but the precise mechanisms involved have not been elucidated. Now, Wang et al. have delineated a key pathway mediating mitochondrion-dependent apoptosis in ischaemia that is regulated by the microRNA (miRNA) miR-499.
Evolutionary adaptations of human hearts
Great apes engage in short bursts of resistance physical activity, such as climbing and fighting, which exerts pressure stress on the cardiovascular system. By contrast, pre-industrial human activity was characterized by moderate-intensity endurance physical activity, such as hunting, gathering and farming, which exerts volume stress. A new study assessing the hearts of chimpanzees, gorillas and humans now shows that the left ventricle of human hearts has derived features that help to augment cardiac output, favouring endurance physical activity. However, the human left ventricle is highly plastic and can remodel in response to chronic pressure or volume stimuli. In response to physical inactivity or sustained pressure loading, the human left ventricle acquires a chimpanzee-like phenotype, which could be a trade-off between pressure adaptations and volume capabilities. The decline in regular moderate-intensity endurance physical activity in post-industrial society could underlie the modern epidemic of hypertensive heart disease.
Cells of the adult human heart
Cardiovascular disease is the leading cause of death worldwide. Advanced insights into disease mechanisms and therapeutic strategies require a deeper understanding of the molecular processes involved in the healthy heart. Knowledge of the full repertoire of cardiac cells and their gene expression profiles is a fundamental first step in this endeavour. Here, using state-of-the-art analyses of large-scale single-cell and single-nucleus transcriptomes, we characterize six anatomical adult heart regions. Our results highlight the cellular heterogeneity of cardiomyocytes, pericytes and fibroblasts, and reveal distinct atrial and ventricular subsets of cells with diverse developmental origins and specialized properties. We define the complexity of the cardiac vasculature and its changes along the arterio-venous axis. In the immune compartment, we identify cardiac-resident macrophages with inflammatory and protective transcriptional signatures. Furthermore, analyses of cell-to-cell interactions highlight different networks of macrophages, fibroblasts and cardiomyocytes between atria and ventricles that are distinct from those of skeletal muscle. Our human cardiac cell atlas improves our understanding of the human heart and provides a valuable reference for future studies.
Stay Up to Date
Join our mailing list to stay on top of Papers’ latest updates.